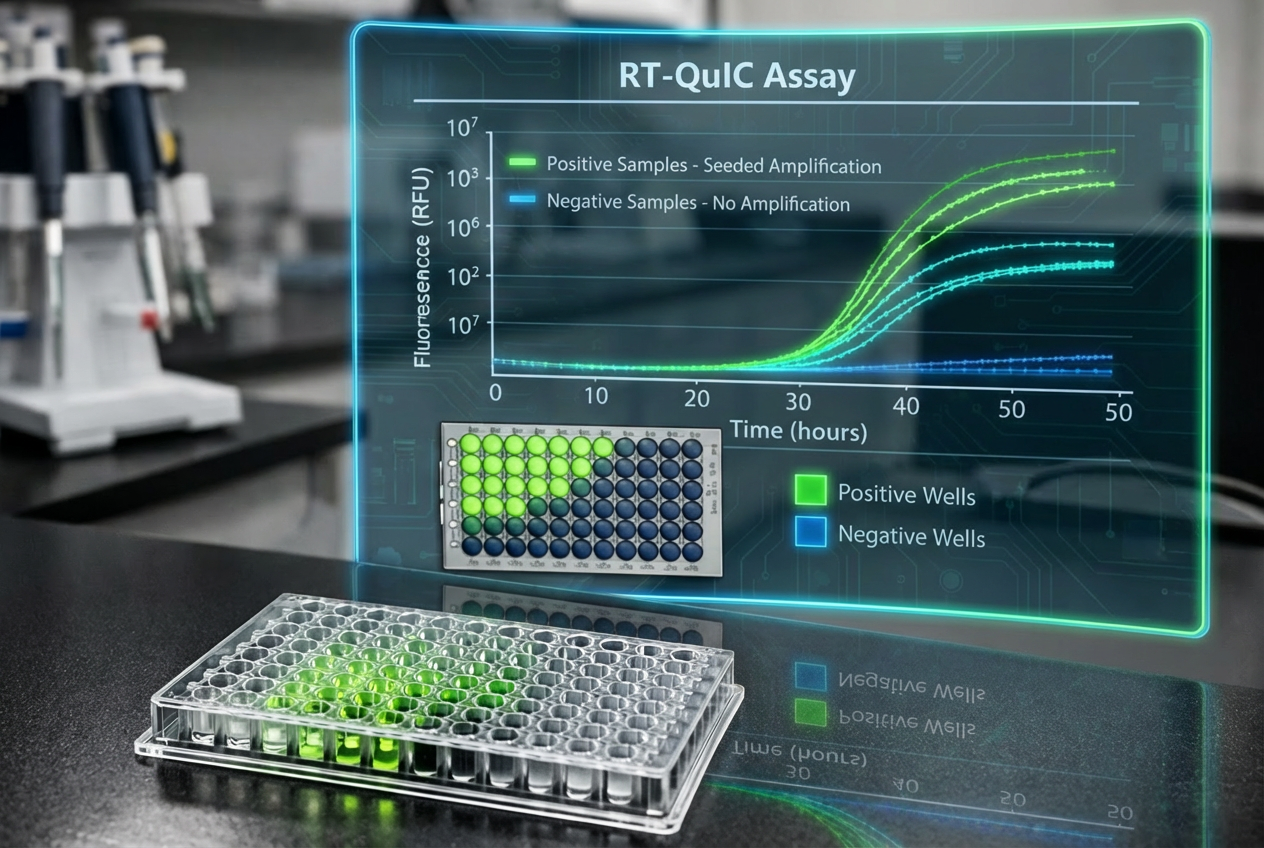
Real‑time Quaking‑Induced Conversion (RT‑QuIC) detects prions—the misfolded proteins behind Chronic Wasting Disease (CWD), Bovine Spongiform Encephalopathy (BSE), and Scrapie—with unprecedented sensitivity and speed. Over the past decade, RT-QuIC has grown to be a powerful test for detecting prions across a variety of sample types.
Why RT‑QuIC Is a Game‑Changer
Traditional prion tests (e.g., ELISA, Western blot) require large sample volumes, can miss low‑level contamination, and are not appropriate for testing some materials. RT‑QuIC amplifies trace amounts of pathogenic protein by converting normal recombinant substrate into the same misfolded shape, producing a measurable fluorescent signal in just a few hours. The result is:
- 1,000‑10,000× higher sensitivity than legacy methods.
- Rapid turnaround.
- Small sample requirement.
How the Test Works (Simplified)
1. Sample preparation – a tiny portion of the finished product is mixed with a buffer that releases any prion particles.
2. Seeded conversion – the mixture is added to a plate containing recombinant prion substrate and a fluorescent dye.
3. Quaking cycle – the plate is shaken (the “quake”) at controlled temperatures, causing any seed‑prions present to convert the substrate into aggregates.
4. Readout – fluorescence grows proportionally with aggregate formation; crossing a predefined threshold indicates a positive result.
RT-QuIC testing provided by Priogen, the leading independent third‑party prion diagnostic lab.
Integration with the Cervid Sure™ Badge
The badge program now leverages RT‑QuIC results directly:
- Each product that passes an RT‑QuIC screen receives a unique QR code.
- Scanning the QR code pulls up the test report.
- The badge is displayed on packaging, marketing material and online listings, instantly signaling “verified no prion detection.”
In the Natural Products Marketplace
Recent studies show it is possible to detect environmental prions if samples are prepared carefully and tested with sensitive amplification assays like RT‑QuIC. Researchers developed a method to extract, concentrate, and detect prions from plant tissues, demonstrating prions on plant surfaces and inside plants. Separate work on soils showed native soil components can cause false signals, so teams created soil‑specific extraction protocols, ran negative controls, and used spiking experiments to confirm detection reliability. Other comparative studies indicate that with proper sample processing and controls, RT‑QuIC and similar assays can detect prions in complex materials such as soil, waterways, and many surfaces. Altogether, these manuscripts provide practical, vetted approaches for finding CWD prions in environmental and product samples and offer guidance for applying the methods to different locations and sample types.
The Bottom Line
RT-QuIC is powerful because it detects tiny amounts of misfolded prion proteins with very high sensitivity and specificity, often earlier and more reliably than older tests. The assay amplifies abnormal protein seeds by repeatedly converting normal substrate proteins into the misfolded form, producing a strong fluorescent signal that’s easy to measure. It works on small samples like cerebrospinal fluid or nasal brushings, is relatively fast compared with lengthy bioassays, and reduces the need for animal testing. Because RT-QuIC targets the biological mechanism of prion replication, it distinguishes disease-associated protein aggregates from harmless forms, making it useful for accurate diagnosis and for screening potential therapies. Overall, its combination of early detection, reliability, speed, and reduced ethical burden makes RT-QuIC a major advance in prion disease testing.